A Clinical Research Study for People with Primary Ciliary Dyskinesia (PCD)
Due to Disease-Causing Mutations in the DNAI1 Gene
The RCT1100-102 clinical research study is exploring a potential new treatment for individuals with PCD due to disease-causing mutations in the DNAI1 gene.
Here, you will find more info about the study, the investigational medicine, and how you, or someone you know, can participate
About PCD
PCD is a rare genetic disorder that leads to chronic respiratory infections and permanent damage to the lungs called bronchiectasis.
PCD affects the cilia, the tiny hair-like structures on the surface of cells that line the upper and lower airways. This leads to mucus and debris building up in the respiratory tract, sinuses, and ears. Consequently, patients may suffer from chronic respiratory infections, chronic ear infections, and more.
Mutations in more than 40 genes result in dysfunctional cilia and loss of mucociliary clearance. PCD is a progressive disorder, meaning it will get worse over time, and no cure currently exists.

About PCD
PCD is a rare genetic disorder that leads to chronic respiratory infections and permanent damage to the lungs called bronchiectasis.
PCD affects the cilia, the tiny hair-like structures on the surface of cells that line the upper and lower airways. This leads to mucus and debris building up in the respiratory tract, sinuses, and ears. Consequently, patients may suffer from chronic respiratory infections, chronic ear infections, and more.
Mutations in more than 40 genes result in dysfunctional cilia and loss of mucociliary clearance. PCD is a progressive disorder, meaning it will get worse over time, and no cure currently exists.

Why this Study is Important
There are currently no approved treatments for PCD.
The goal of this study is to find out whether the investigational study medicine is safe and may work as a potential new treatment option for those who have PCD due to disease-causing mutations in the DNAI1 gene.
To learn more, contact:
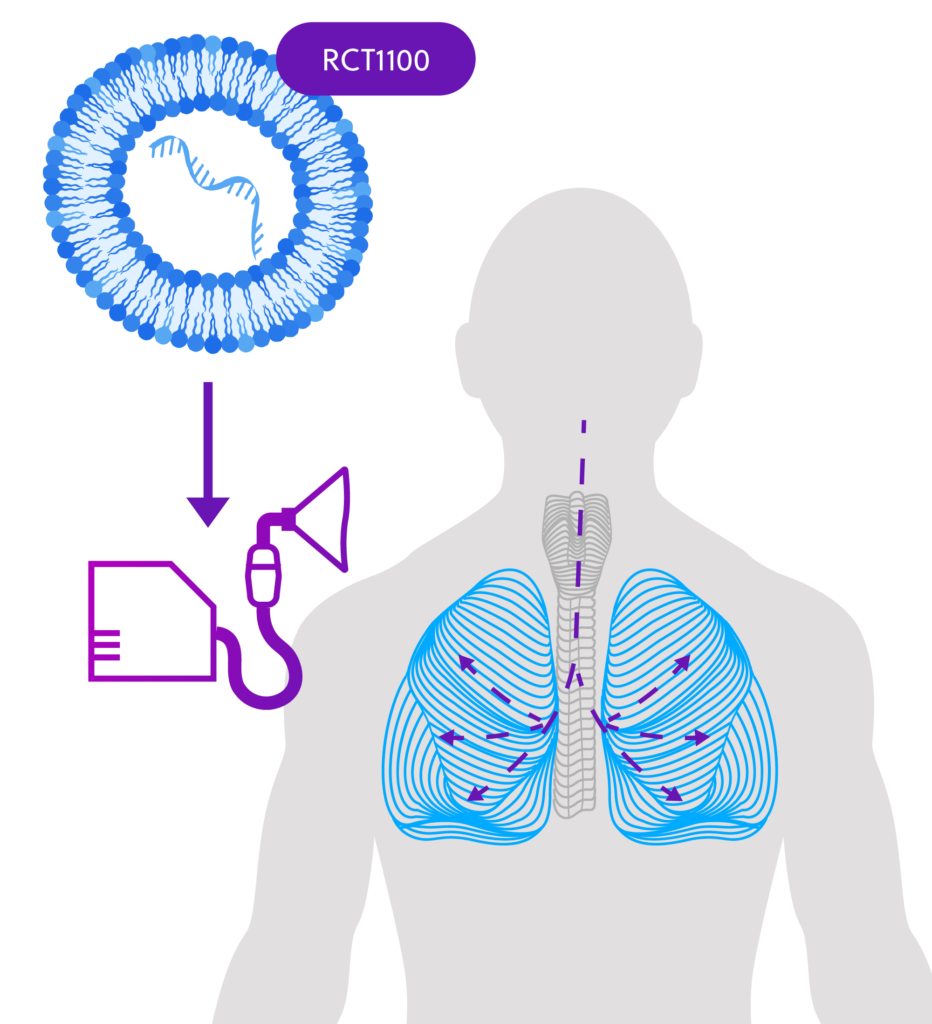
How the Study Medicine Works
ReCode is developing RCT1100, an investigational messenger RNA (mRNA)-based therapeutic for PCD caused by disease-causing mutations in DNAI1, a gene responsible for ciliary movement. About 10% of people with PCD are missing the DNAI1 protein.
By giving DNAI1-mRNA as a therapy to people with PCD caused by this missing protein, it may be possible to restore ciliary function in the lungs, addressing the underlying cause of PCD rather than just controlling the symptoms of the disease.
In a Phase 1 single-dose study, RCT1100 was well tolerated by healthy individuals and a small number of people with PCD with disease-causing mutations in DNAI1.
How the Study Medicine is Given
The investigational study medicine, RCT1100, will be given using a nebulizer — a medical device that converts liquid medication into a mist for inhalation.
1 Screening
During the screening period, you will receive an initial consultation with the study doctor to assess your suitability for the study, and then, if not previously performed in study RCT1100-101, a bronchoscopy will be conducted at the hospital.
2 Treatment
If you are eligible, you will receive an inhaled dose of RCT1100 3 times a week for 12 weeks (which may be reduced to twice a week).
- If eligible, a bronchoscopy procedure will be conducted during screening.
- First Dose The first dose will be given in the clinic, and you will stay overnight to be observed.
- The Second, Third, and Fourth Doses These doses will also be given in the outpatient clinic, and you will be discharged after being observed for approximately one hour.
- All Other Doses Can be given at the outpatient clinic or at home by a qualified nurse. You will visit the clinic for study procedures at Weeks 4, 8, & 12.
- You will have a second bronchoscopy conducted at Week 4 or Week 12.
3 Follow-up
There will be a 12-week follow-up period, which includes a final outpatient visit approximately three months after your last dose.
Home Dosing Visits
During your participation in the clinical trial, if your study doctor approves, you may be given the option to have some of your visits carried out at your home by a qualified and experienced Research Nurse. The nurses will provide you with all the details of the visit and consult with you about the time and date of your visit. They will also report the details of your visit to your study doctor. You may reach out to your study doctor with any concerns regarding your Research Nurse and decide to stop the home visits at any time.
Study tests and procedures
Throughout the study, you will have certain exams and tests, including:
Physical Exam
to check your health, height and weight
Vital Signs Measurement,
including blood pressure, pulse rate
(if you have not had within the past year)
Bronchoscopy*
A thin, flexible tube will be inserted through the nose or mouth to examine the airways and lungs and collect small tissue samples.
Nasal Brushings
to obtain a sample of cilia (pre-dose and taken at the time of bronchoscopy unless already obtained from a prior study with RCT1100)
Physical Exam
to check your health, height and weight
Vital Signs Measurement,
including blood pressure, pulse rate
(if you have not had within the past year)
A thin, flexible tube will be inserted through the nose or mouth to examine the airways and lungs and collect small tissue samples.
Blood and Urine Samples
to monitor your safety, measure how much RCT1100 is in your blood, and measure the effect of RCT1100 on your immune system
to measure lung function
assessments to see how much nitric oxide you exhale
which record the electrical signal from the heart
Doing exercises to help loosen any sputum (mucus, phlegm). You will be asked to try and cough to clear your throat.
to obtain a sample of cilia (pre-dose and taken at the time of bronchoscopy unless already obtained from a prior study with RCT1100)
to monitor your safety, measure how much RCT1100 is in your blood, and measure the effect of RCT1100 on your immune system
to measure lung function
assessments to see how much nitric oxide you exhale
Electrocardiograms
which record the electrical signal from the heart
Doing exercises to help loosen any sputum (mucus, phlegm). You will be asked to try and cough to clear your throat.
*This procedure is done twice for all participants: at the start of the study and at Week 4 or 12. It is conducted either under sedation with local anesthesia or with a general anesthetic.
Eligibility
- Those who are 18-70 years old
- Those with a clinical diagnosis of PCD
- People with PCD due to disease-causing mutations in the DNAI1 gene
- Those with predicted forced expiratory volume of at least 50% (with bronchodilators not given for 4-12 hours)
- People with any other clinically significant medical condition that may impact your safety
- Those with a history of cancer, except adequately treated skin cancers
- Those who have a bleeding risk
- People currently on anticoagulation therapy
- Anyone who has received the COVID vaccine or live virus vaccine within 2 weeks of treatment or plan to receive a COVID vaccine within 6 weeks after the last treatment
- Those with a recent history of alcohol abuse, drug addiction, or active smoker
- Anyone who has had major trauma or surgery within 4 weeks of screening
- Anyone who has received an investigational product other than RCT1100 within a certain period
- Those who are pregnant or breastfeeding
FAQs
What is a clinical research study?
Where will this study take place?
The study will occur at the Royal Brompton Hospital, the largest specialist heart and lung medical centre in the United Kingdom. If you decide to participate in the clinical research study, you will receive detailed information regarding the study location and any possible home visits.
How much of my time will this clinical research study take up?
If you are eligible and decide to participate in the study, a study doctor will share more information about the number of visits and the time expected from you during the study.
- There are three parts to the study
- Part 1 is the screening period, which takes up to 28 days
If eligible, a bronchoscopy procedure will be conducted during screening. - Part 2 is the treatment period, which will take 12 weeks. Your second bronchoscopy will be either at Week 4 or 12
- Part 3 is the follow-up period, which will also take 12 weeks
- Part 1 is the screening period, which takes up to 28 days
- If you participate, you can expect to be in the study for about 28 weeks (~7 months).
Will I receive compensation for participating in the study?
You will be reimbursed for completion of the study.

